kimlab
Iron and Cardiovascular Disease
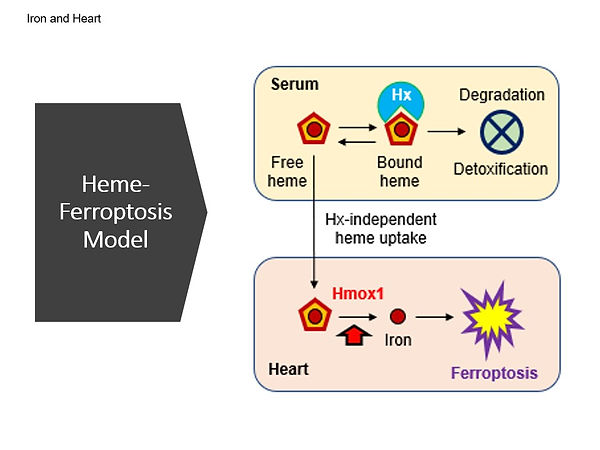
Iron is an essential nutrient metal, but too much iron is toxic. We have been studying the molecular mechanisms underlying cardiac toxicity in iron overload disorders, such as hemochromatosis and sickle cell disease. We identified detrimental effects of iron overload on the heart, which can lead to age-associated cardiac hypertrophy and fibrosis. We also investigated the role of excess heme and ferroptosis in the development of cardiac complications in sickle cell disease. Furthermore, we recently demonstrated that iron promotes intracellular accumulation of doxorubicin, an anthracycline chemotherapeutic agent, in the heart and increases cardiac toxicity through downregulation of the mitochondrial exporter ABCB8. In addition, hemopexin, a protein that binds and transports heme, may modulate anthracycline-induced cardiotoxicity in both patients and mice. The findings from our studies suggest that targeting ferroptosis and reducing iron accumulation in the heart could be potential therapeutic strategies to prevent or treat heart failure in patients with iron overload. Additionally, hemopexin may be a potential therapeutic target for preventing or mitigating anthracycline-induced cardiotoxicity. Together, our studies underscore the importance of understanding the underlying mechanisms of cardiac complications in hemochromatosis, sickle cell disease, and anthracycline therapy. By identifying therapeutic targets and approaches, our research has the potential to improve the health outcomes of patients with these conditions.
References:
Menon AV, Liu J, Tsai HP, Zeng L, Yang S, Asnani A, Kim J. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood 2022; 139(6):936-941. PMID: 34388243.
Liu J, Lane S, Lall R, Russo M, Farrell L, Debreli Coskun M, Curtin C, Araujo-Gutierrez R, Scherrer-Crosbie M, Trachtenberg BH, Kim J, Tolosano E, Ghigo A, Gerszten RE, Asnani A. Circulating hemopexin modulates anthracycline cardiac toxicity in patients and in mice. Sci Adv 2022; 8(51):eadc9245. PMID: 36563141.
Menon AV, Kim J. Iron promotes cardiac doxorubicin retention and toxicity through downregulation of the mitochondrial exporter ABCB8. Front Pharmacol 2022; 13:817951. PMID: 35359834.
Sukumaran A, Chang J, Han M, Mintri S, Khaw BA, Kim J. Iron overload exacerbates age-associated cardiac hypertrophy in a mouse model of hemochromatosis. Sci Rep 2017; 7:5756. PMID: 28720890.
Iron and Brain Health
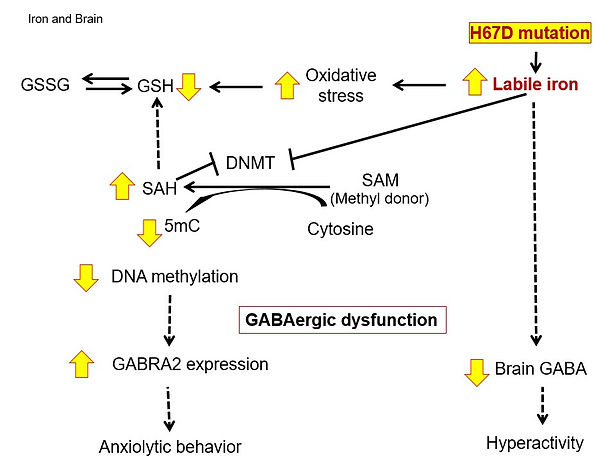
Iron is required for proper brain development and function, including myelination, neurotransmitter synthesis, and electron transfer. While iron deficiency is closely associated with altered neurotransmitter metabolism and affective behavior, excess iron is neurotoxic because of its ability to increase oxidative stress. We have been studying the role of brain iron and metal metabolism in the pathogenesis of neurodegenerative diseases, mental disorders, and neurological dysfunction. For example, we demonstrated that brain iron loading increases oxidative stress, impairs DNA methylation and alters GABAergic function in mice, resulting in anxiety-like behavior. We also showed that loss of divalent metal transporter 1 (DMT1) function promotes copper accumulation in the brain, impairs GABA function, and increases impulsivity. These studies suggest that abnormal metabolism of brain metals could contribute to affective disorders and provide insights into potential therapeutic strategies for these disorders, such as metal chelation therapy. Indeed, we found that treatment of iron chelators that enter the brain effectively reverses iron accumulation and decreases oxidative stress in the brain of mice with brain iron overload. Our research demonstrates that normalizing high brain iron provides a neuroprotective effect against iron-associated CNS dysfunction, including neuropsychiatric disorders and neurodegenerative diseases.
References:
Cheng R, Dhorajia V, Kim J, Kim Y. Mitochondrial iron metabolism and neurodegenerative diseases. Neurotoxicology 2022; 88:88-101. PMID: 34748789.
Cheng R, Gadde R, Fan Y, Kulkarni N, Shevale N, Bao K, Choi HS, Betharia S, Kim J. Reversal of genetic brain iron accumulation by N,N’-bis(2-mercaptoethyl)isophthalamide, a lipophilic metal chelator, in mice. Arch Toxicol 2022; 96(7):1951-1962. PMID: 35445828.
Ye Q, Trivedi M, Zhang Y, Böhlke M, Alsulimani HH, Chang J, Maher TJ, Deth RC, Kim J. Brain iron loading impairs DNA methylation and alters GABAergic function in mice. FASEB J 2019; 32:2460-2471. PMID: 30277817.
Han M, Chang J, Kim J. Loss of divalent metal transporter 1 (DMT1) function promotes brain copper accumulation and increases impulsivity. J Neurochem 2016; 138:918-928. PMID: 27331785.
Han M, Kim J. Effect of dietary iron loading on recognition memory in growing rats. PLoS One 2015; 10:e0120609. PMID: 25746420.
Manganese and Neurotoxicity
Environmental and occupational exposure to heavy metals remains one of the major concerns in public health. Increased levels of manganese (Mn) are associated with profound neurotoxic effects, including neurobehavioral deficits and disturbances that resemble Parkinson’s disease. High levels of Mn in the brain can be achieved via inhalation due to direct transport into the brain, which bypasses hepatic clearance of Mn after oral ingestion. We have been studying the effects of Mn exposure on brain function and behavior, specifically in relation to neurotoxicity, memory impairment and motor dysfunction, in the context of iron metabolism and gene-environment-lifestyle interaction. For example, we investigated the influence of iron overload hemochromatosis on neurobehavioral performance after Mn exposure. We found that hemochromatosis exacerbates Mn-induced motor dysfunction and impairs recognition memory after Mn exposure by drinking water. In contrast, hemochromatosis improves anxiety and impaired memory function induced by airborne Mn exposure, suggesting that iron overload can modify Mn neurotoxicity in an exposure route-dependent manner. In addition, we earlier explored the impact of olfactory Mn exposure on low iron status and found that iron deficiency increases Mn transport into the brain, which surprisingly reverses abnormal dopamine metabolism and motor impairment caused by low iron. This suggests that Mn could mimic iron and support iron-dependent enzymes when iron is low in the brain. In addition, we have investigated the impact of alcohol drinking on Mn-induced neurotoxicity in mice. Our results demonstrated that alcohol consumption up-regulates iron transporters that also transfer Mn into the brain after intranasal administration of Mn, thereby increasing brain Mn levels and impairing dopaminergic function. This study suggests that individuals who consume alcohol may have a higher risk of Mn neurotoxicity upon Mn inhalation. Our research provides valuable insights into the complex interplay between Mn and iron in the brain, highlighting the potential for these metals to impact brain function and behavior.
References:
Han M, Böhlke M, Maher TJ, Kim J. Alcohol exposure increases manganese accumulation in the brain and exacerbates manganese-induced neurotoxicity in mice. Arch Toxicol 2021; 95(12):3665-3679. PMID: 34590183.
Ye Q, Park JE, Gugnani K, Betharia S, Pino-Figueroa A, Kim J. Influence of iron metabolism on manganese transport and toxicity. Metallomics 2017; 9:1028-1046. PMID: 28620665.
Ye Q, Kim J. Mutation in HFE gene decreases manganese accumulation and oxidative stress in the brain after olfactory manganese exposure. Metallomics 2016; 8: 618-627. PMID: 27295312.
Alsulimani HH, Ye Q, Kim J. Effect of Hfe deficiency on memory capacity and motor coordination after manganese exposure by drinking water in mice. Toxicol Res 2015; 31(4): 347-354. PMID: 26877837.
Ye Q, Kim J. Effect of olfactory manganese exposure on anxiety-related behavior in a mouse model of hemochromatosis. Environ Toxicol Pharmacol 2015; 40(1):333-341. PMID: 26189056.
Ye Q, Kim J. Loss of Hfe function reverses impaired recognition memory caused by olfactory manganese exposure in mice. Toxicol Res 2015; 31(1):17-23. PMID: 25874029.
Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS One 2013; 8:e64944. PMID: 23705020.
Kim J, Li Y, Buckett PD, Böhlke M, Thompson KJ, Takahashi M, Maher TJ, Wessling-Resnick M. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PLoS One 2012; 7:e33533. PMID: 22479410.